
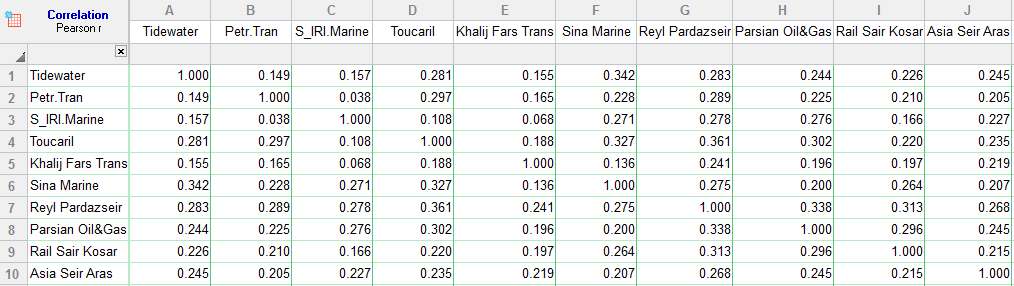
You see them showing gene expression, phylogenetic distance, metabolomic profiles, and a whole lot more. They are an intuitive way to visualize information from complex data. We also confirm that fatty acid uptake and oxidation are targetable metabolic dependencies in human prostate cancer.Heatmap, heatmap everywhere. These findings demonstrate that human prostate cancer, irrespective of disease stage, can effectively utilize all metabolic substrates, albeit with marked heterogeneity across tumors. Blocking fatty acid uptake or fatty acid oxidation with pharmacologic inhibitors was sufficient to reduce cell viability in PDX-derived organoids, whereas blockade of DNL, or glucose or glutamine oxidation induced variable and limited therapeutic efficacy. Mechanistically, glucose utilization was mediated by acetyl-CoA production rather than carboxylation of pyruvate, while glutamine entered the tricarboxylic acid cycle through transaminase reactions before being utilized via oxidative or reductive pathways. There was no difference in substrate utilization between localized and metastatic PDXs and hierarchical clustering revealed marked metabolic heterogeneity across all PDXs. De novo lipogenesis (DNL) and storage of free fatty acids into phospholipids and triacylglycerols were increased in malignant PDXs. Glucose, glutamine, and fatty acid oxidation was variably upregulated in malignant PDXs compared with benign PDXs.


To address this knowledge gap, we performed radiometric ( 14C) and stable ( 13C) isotope tracing assays in precision-cut slices of patient-derived xenografts (PDX). Studies in cells and mice have highlighted the importance of oxidative metabolism and lipogenesis in prostate cancer however, the metabolic landscape of human prostate cancer remains unclear. Cancer cells undergo metabolic reprogramming to meet increased bioenergetic demands.


 0 kommentar(er)
0 kommentar(er)
